Abstract
Introduction: CML is a chronic myeloproliferative neoplasm that is characterized by presence of Philadelphia chromosome (a translocation between chromosomes 9 and 22 leading to BCR-ABL1 fusion gene and protein). This fusion can be detected by karyotyping, FISH and polymerase chain reaction (PCR). The therapy of CML has evolved from hydroxyurea, interferon, allogeneic stem cell transplant and lately tyrosine kinase inhibitors (TKI). The target of therapy is molecular response (MR) and has also evolved from the major molecular response (MMR), defined as ≥3-log reduction in BCR/ABL1 fusion transcript using real-time quantitative PCR (RQ-PCR) to deep molecular response (DMR, ≥4-log reduction). The experience from a number of clinical trials, including STIM, TWISTER, A-STIM and KIDS, has generated interest in stopping TKIs in patients who have reached a sustained DMR. Hughes et al (Blood 2016) have published practical guidelines for selection of candidates for TFR and their subsequent management. Here we report our experience with TFR in patients with CML in DMR.
Method: We searched our database for CML patients on continuous TKI therapy and monitoring. Patient not followed for more than 6 months were excluded from analysis. Molecular response was monitored in all patients at intervals defined by National Comprehensive Centre Network (NCCN) guidelines (available at www.nccn.org). Peripheral blood samples were collected in Tempus™ Blood RNA Tubes and analysed in a central Molecular Diagnostics Laboratory. Real-time quantitation of BCR-ABL1 gene transcripts was performed using the Qiagen-Ipsogen BCR-ABL1 Mbcr IS-MMR Dx® kit. The results were expressed as a percent ratio of BCR-ABL1 to ABL1 transcripts on the International Scale (IS). The assay can quantitate up to 4.96 log reductions (0.0011%IS) calculated according to the recommendations of Cross et al (Leukemia 2015).
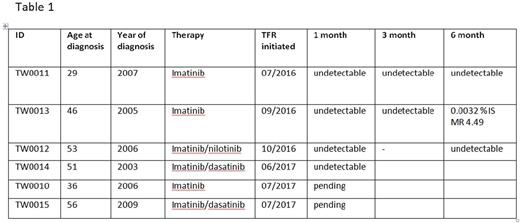
Result: We identified One hundred and seventeen CML patients on continuous TKI therapy and monitoring in our database. The median age was 36 years (range, 15-73 years). In our cohort 66/117 (56%) had achieved a DMR, while 20/117 (17%) had achieved undetectable levels of fusion transcript (≥4.5-log reduction, MR4.5). Fifteen out of the twenty (75%) with MR4.5 were candidates for TFR as they fulfilled the criteria proposed by Hughes et al (Blood 2016) of being more than 8 years on therapy with optimal response. Nine patients were counselled about TFR, monitoring and definition of progression and risk of progression. Six patients from this cohort have been initiated on TFR (Table 1). The median period of TKI therapy was 11 years. All six had undetectable levels of BCR-ABL1 transcript for more than 2 years. All were followed monthly except for the patient TW0012. Three patients have reached the six month milestone with one patient having MR 4.49 while the other 2 remain at undetectable levels. None of the patients experienced TKI withdrawal syndrome (myalgia/arthralgia) or expressed psychological anxiety for relapse.
Conclusion: TFR is a very important goal especially in a young patient population. Continuous and chronic TKI therapy not only adds economic burden to the patient and healthcare system, but can also have undesirable side effects. Our limited experience outside of a clinical trial and in real world shows that willing and compliant patients and a reliable molecular laboratory will be the cornerstones of TFR as the future goal of CML.
As our confidence builds with TFR, we anticipate extending it to candidates with MR4.5 when they reach 8 years or more of TKI therapy threshold.
Alam: BMS: Honoraria; Biologix: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal